| SKU | Stock | Quantity (gr) | Price |
|---|---|---|---|
| Actu255-50G | In stock | 500 | €50 |
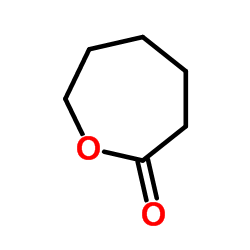
Caprolactone or simply caprolactone is a cyclic ester, a member of the lactone family, with a seven-membered ring with the formula (CH2)5CO2. This colorless liquid is miscible with most organic solvents. It is produced on a large scale as a precursor to polycaprolactones. The major use of caprolactone is the production of polycaprolactones. These are split into two categories; the production of low molecular weight polycaprolactone polyols, used in specialty polyurethanes and coatings, and high molecular weight thermoplastics used in a variety of applications. There is published usage for caprolactone is its conversion to caprolactam, billions of kilograms of which are produced annually but not by this route.
Carbonylation of caprolactone gives, after hydrolysis, pimelic acid. The lactone ring is easily opened with nucleophiles including alcohols and water to give polylactones and eventually the 6-hydroxyadipic acid.
Aliphatic polyesters II. The degradation of poly (DL-lactide), poly (-caprolactone), and their copolymers in vivo
G.G. Pitt, M.M. Gratzl, G.L. Kimmel, J. Surles, A. Sohindler
The mechanisms of biodegradation of poly (DL-lactide), poly(Ɛ-caprolactone), and copolymers of caprolactone with DL-dilactide, valerolactone, and DL-decalactone in rabbit were shown to be qualitatively similar. However, the rate of the first stage of the degradation process, non-enzymatic random hydrolytic chain scission, varied by an order of magnitude and was dependent on morphological as well as chemical effects. Weight loss was generally not observed until the molecular weight had decreased to 15 000 or less. Poly (DL-lactide) differed from the other polyesters studied, the rate of chain scission increasing after the commencement of weight loss. The rate of weight loss was greater and the period prior to weight loss was shorter when the comonomer content of copolymers of Ɛ-caprolactone was sufficient to reduce the melting point of Ɛ-caproate sequences to body temperature.
Polymer-layered silicate nanocomposites: in situ intercalative polymerization of .epsilon.-caprolactone in layered silicates
Phillip B. Messersmith, Emmanuel P. Giannelis
No abstract available
Poly-Ɛ-caprolactone microspheres and nanospheres: an overview
V.R. Sinha, K. Bansal, R. Kaushik, R. Kumria, A. Trehan
Poly-caprolactone (PCL) is a biodegradable, biocompatible and semicrystalline polymer having a very low glass transition temperature. Due to its slow degradation, PCL is ideally suitable for long-term delivery extending over a period of more than one year. This has led to its application in the preparation of different delivery systems in the form of microspheres, nanospheres and implants. Various categories of drugs have been encapsulated in PCL for targeted drug delivery and for controlled drug release. Microspheres of PCL either alone or of PCL copolymers have been prepared to obtain the drug release characteristics. This article reviews the advancements made in PCL-based microspheres and nanospheres with special reference to the method of preparation of these and their suitability in developing effective delivery systems.
Aliphatic polyesters. I. The degradation of poly(Ɛ- caprolactone) in vivo
C.G. Pitt, F.I. Chasalow, Y.M. Hibionada, D.M. Klimas, A. Schindler
The degradation of poly(Ɛ-caprolactone) in rabbits, rats, and water was studied by measurement of changes in intrinsic viscosity, molecular weight, crystallinity, Young’s modulus, and weight. Degradation proceeds by nonenzymatic random hydrolytic cleavage of ester linkages. The process is autocatalytic, and the kinetic relationship Mn/Mno = exp(kt) is observed until the Mn has decreased to approximately 5000. Significant weight loss is not observed until this point but, once initiated, the rate of weight loss depends markedly on the particle size. Chain scission is associated with an increase in crystallinity, which partly determines the rate of degradation.
Caprolactone or simply caprolactone is a cyclic ester, a member of the lactone family, with a seven-membered ring with the formula (CH2)5CO2. This colorless liquid is miscible with most organic solvents. It is produced on a large scale as a precursor to polycaprolactones. The major use of caprolactone is the production of polycaprolactones. These are split into two categories; the production of low molecular weight polycaprolactone polyols, used in specialty polyurethanes and coatings, and high molecular weight thermoplastics used in a variety of applications. There is published usage for caprolactone is its conversion to caprolactam, billions of kilograms of which are produced annually but not by this route.
Carbonylation of caprolactone gives, after hydrolysis, pimelic acid. The lactone ring is easily opened with nucleophiles including alcohols and water to give polylactones and eventually the 6-hydroxyadipic acid.
Aliphatic polyesters II. The degradation of poly (DL-lactide), poly (-caprolactone), and their copolymers in vivo
G.G. Pitt, M.M. Gratzl, G.L. Kimmel, J. Surles, A. Sohindler
The mechanisms of biodegradation of poly (DL-lactide), poly(Ɛ-caprolactone), and copolymers of caprolactone with DL-dilactide, valerolactone, and DL-decalactone in rabbit were shown to be qualitatively similar. However, the rate of the first stage of the degradation process, non-enzymatic random hydrolytic chain scission, varied by an order of magnitude and was dependent on morphological as well as chemical effects. Weight loss was generally not observed until the molecular weight had decreased to 15 000 or less. Poly (DL-lactide) differed from the other polyesters studied, the rate of chain scission increasing after the commencement of weight loss. The rate of weight loss was greater and the period prior to weight loss was shorter when the comonomer content of copolymers of Ɛ-caprolactone was sufficient to reduce the melting point of Ɛ-caproate sequences to body temperature.
Polymer-layered silicate nanocomposites: in situ intercalative polymerization of .epsilon.-caprolactone in layered silicates
Phillip B. Messersmith, Emmanuel P. Giannelis
No abstract available
Poly-Ɛ-caprolactone microspheres and nanospheres: an overview
V.R. Sinha, K. Bansal, R. Kaushik, R. Kumria, A. Trehan
Poly-caprolactone (PCL) is a biodegradable, biocompatible and semicrystalline polymer having a very low glass transition temperature. Due to its slow degradation, PCL is ideally suitable for long-term delivery extending over a period of more than one year. This has led to its application in the preparation of different delivery systems in the form of microspheres, nanospheres and implants. Various categories of drugs have been encapsulated in PCL for targeted drug delivery and for controlled drug release. Microspheres of PCL either alone or of PCL copolymers have been prepared to obtain the drug release characteristics. This article reviews the advancements made in PCL-based microspheres and nanospheres with special reference to the method of preparation of these and their suitability in developing effective delivery systems.
Aliphatic polyesters. I. The degradation of poly(Ɛ- caprolactone) in vivo
C.G. Pitt, F.I. Chasalow, Y.M. Hibionada, D.M. Klimas, A. Schindler
The degradation of poly(Ɛ-caprolactone) in rabbits, rats, and water was studied by measurement of changes in intrinsic viscosity, molecular weight, crystallinity, Young’s modulus, and weight. Degradation proceeds by nonenzymatic random hydrolytic cleavage of ester linkages. The process is autocatalytic, and the kinetic relationship Mn/Mno = exp(kt) is observed until the Mn has decreased to approximately 5000. Significant weight loss is not observed until this point but, once initiated, the rate of weight loss depends markedly on the particle size. Chain scission is associated with an increase in crystallinity, which partly determines the rate of degradation.
Please request this information: info@4medchem.com.
Please request this information: info@4medchem.com.
Please request this information: info@4medchem.com.