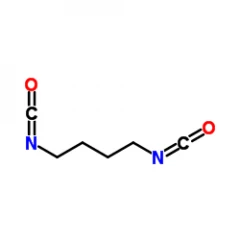
Purity: 99,7%
Cas: 4538-37-8
Synonyms: 1,4-Butane diisocyanate | 1,4-butanediisocyanate | 1,4-diisocyanatobutane | butane-1,4-diisocyanate | Tetramethylene diisocyanate
MDL: MFCD00002968
Boiling point: 230 C (lit.)
Density: 1,017g/ml at 25 C (lit)
Metling T: 16 C (lit)
| Cat nr | Stock | Quantity (gr) | Price |
| 8040403005 | Out of stock | 5 g | €125 |
| 8040403010 | In stock | 10 g | €175 |
| 8040403100 | In stock | 100 g | €480 |
| 8040403500 | In stock | 500 g | €1490 |
| 80404031000 | In stock | 1000 g | €2350 |
**Pricing disclaimer
1,4-Diisocyanatobutane ( Cas 4538-37-8) was used in over 8 studies because of its proved non-toxic effects on human health. These studies comprehend early research studies about biodegradability/ biocompatibility, In-Vivo studies, and several field studies. Importantly is to take into account the quality of the product as prelimanary starting materials or catalysts could have an influence on the outcome of a research project. As a result we offer a purity of 99,7% 1,4-diisocyanatobutane.
Index
The potential of 1,4-butanediisocyanate (BDI) was already known in 1998 by C.J. Spaans et al. who produced a polyurethane based on ε-caprolactone and 1,4- butanediisocyanate. Through chain extension with a urethane diol block these scientists produced porous polymer which was attractive for its use in meniscal prosthesis. [1] That BDI is excellent for in-vivo applications through its biocompatibility and mechanical properties was stated once more by E. Wisse et al. (2006). [2]
3 Year in-vivo study It was not until 2007 that a 3 year in-vivo study truly proved the biocompatibility of BDI. This study prone performed by B.V. Minnen et al. (2007) showed that highly porous polyurethane (PU) discs made out of copolyester soft segments of DL-lactide/ Ɛ-caprolactone and hard segments synthesized from 1,4- butanediisocyanate is safely used as a biodegradable implant. The porous foam scaffolds were implanted subcutaneously in both rats and rabbits. Interval experiments justified that after 3 years the PU foam discs were resorbed and biocompatible upon degradation. [3]
Drug delivery systems Another application of BDI in PU polymers was proved by A.R. Hafeman et al. (2008). PU polymers made out of BDI, poly(ε-caprolactone) diol, and putrescine were excellent biodegradable elastomeric PU scaffolds for soft tissue engineering applications. The PU drug delivery systems had a two stage release where the first part of the drug was released on day 1 (19%-37%), and the second release was spread over a period of 4 weeks. [4]
Soft tissue engineering, Cell scaffolds in cardiovascular tissue engineering Guan et al. (2004) proved that PU polymers with BDI in the hard segment were very suitable for soft tissue engineering where scaffold must comply with high elastance, strength, and a controllable biodegradability. Therefore a poly(ester)urethane and poly(ester-ester)urethane urea polymer was synthesized with both consisting of a part BDI. Both polymers where processed to flexible scaffolds which proved their high potential as cell scaffolds in for cardiovascular tissue engineering and other possible soft tissue engineering applications. [5]
Tissue engineering, bone tissue development Opposed to soft tissue engineering, the group of S. Guelcher et al. (2007) proved excellent abilities of BDI in tissue engineering applications of bone tissue development. Poly (ester) urethane urea polymers consisting out of BDI confirmed the ability of these polymers having a biomaterial modulus excellent for bone tissue development. [6]
One of the reasons that diphenylmethane-4-4’-diisocyanate (MDI), hydrogenated MDI, toluenediisocyanate (TDI), hexamethylene diisocyanate (HDI) are commonly used is their low volatility and their excellent properties. In addition, there is a great amount of information about their usability in biomedical applications. For biomedical applications one can distinguish two different groups, non-degradable and degradable biocompatible materials. [7]Biodegradability of polycarbonate PU’s through monocyte-derived macrophage esterase is highly dependent on the hard-segment part of the polymers. Polymers without hydrogen bonds would be more prone to hydrolysis, from weak to strong: non-hydrogen bonded carbonate < non-hydrogen bonded urethane < hydrogen bonded carbonate < hydrogen bonded urethane. Thereby J. Santerre et al. (2005) indicates that hydrogen bonding is an important factor in biostable PU materials. [8] The latter effect was confirmed by J. Yin (2012) who stated that an increased amount of hydrogen bond acceptor and donor groups would improve the thermal and mechanical properties of PU polymers. [7]This effect would explain the findings of S. Guelcher et al. (2007) who described a microphase-mixed behaviour with two-component polyurethanes prepared from LDI. Hydrogen bonding would be inhibited between the hard segments of neighbouring polymers which is suggested to result in affecting the mechanical properties of the polymers. [9] The asymmetric hard segment of LDI polyurethanes would significantly hinder ordering soft and hard segments of the polymers. As a reason materials from polyurethanes prepared with LDI would have a lower modulus. [10]
| HDI | BDI | LDI |
 |
 |
 |
Figure 1, Hexamethylene diisocyanate (HDI), lysine-diisocyaante (LDI), and butanediisocyanate (BDI). Stated in paragraph one of this chapter HDI is one of the diisocyanates which is able to generate superior materials. However, upon degradation HDI would release toxic diamines which will be discussed in the next chapter. Like HDI, BDI is a symmetric monomer which would enable to create polyurethanes with superior properties. S. Guelcher et al. (2007) synthesized two segmented, biocompatible, polyurethane elastomers using BDI and LDI. Both polyurethane polymers were tested on their properties with a tyramine- and tyrosine-based chain extender. The BDI polyurethane polymer with tyrosine-based chain extender lowered the modulus by an order magnitude compared to the BDI polyurethane tyramine-based chain extension however was still higher than both LDI prepared polyurethanes. Suggested was the improved micro behaviour of the symmetric BDI polymers would greatly enhance mechanical properties of the polymers. [9]
Figure 2, hydrogen bonding between polyurethane polymers prepared with hard segment BDI. [2]
The commonly commercial available diisocyanates MDI, hydrogenated-MDI, toluene diisocyanate (TDI), and HDI are widely used in biomedical applications however, are also well known about their release of toxic and carcinogenic diamines upon degradation. Although the aforementioned diisocyanates are used for biostable materials, it is yet a discussion which concentration could result in physiological issues. [7] As a result the focus has turned towards two aliphatic diisocyanates which upon degradation result in naturally occurring substances. 1,4-Butanediisocyanate (BDI) and lysine diisocyanate (LDI) are proven to be excellent and non-toxic alternatives. The diamines generated upon degradation from these two diissocyanates are lysine (for LDI) and putrescine, spermidine, and spermine (for BDI). [7] Figure 3 shows all degrading products off all known polyisocyanates. [11]
Both LDI and BDI prepared polyurethanes are promising polymers for biomedical applications In addition, their non-toxic chemistry is a promising feature for their role in biomedical materials. [11] Whereas LDI is already a highly used monomer, BDI has also shown promising features. The main advantage of BDI over LDI is its symmetry. Recent publications have proven that polyurethanes prepared with BDI are excellent for bone tissue engineering and soft tissue engineering like cardiovascular tissue engineering. As there more academia become aware of the abilities with BDI the industry still has to follow-up.
| Monomer | Chemical structure | Degradation remarks |
| BDI (butane diisocyanate) | 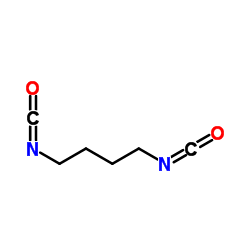 |
Results in putrescine, a naturally occuring product which enhances cell growth and differentiation. This effect is advantageous for tissue engineering, regeneration, and other biomedical applications |
| HDI (hexamethylene diisocyanate) | 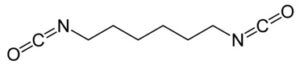 |
Upon degradation diamines are released, a product which is noted to be toxic for several human organs such as the liver and kidney. |
| IPDI (Isophorone diisocyanate) | 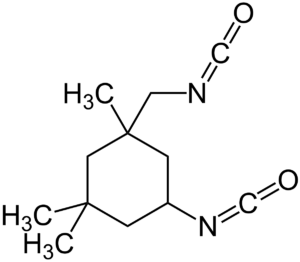 |
The degradtion product of IPDI is less toxic than aromatic isocayanates. |
| LDI (lysine diisocyanate) | 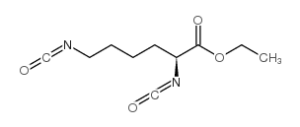 |
Upon degradation LDI would degrade into lysine, a non-toxic by-product. |
| MDI (methylenebis (phenyl isosocyanate)) | 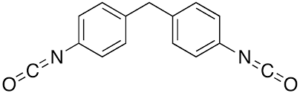 |
Toxic aromatic diamine degradation products |
| TDI (toluene diisocyanate) | 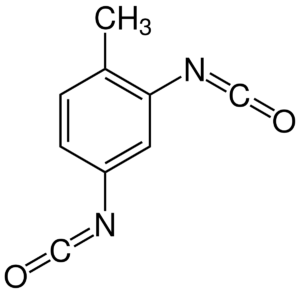 |
Like other other aromatic diisocyanates TDI has been noted to release aromatic diamines, a toxic degradation product known to stimulate carcigogenesis and mutagenesis. |
Figure 3, degradation of polyisocyanates.
[1] Spaans, C. J., Groot, J. H., Dekens, F. G., & Pennings, A. J. (1998). High molecular weight polyurethanes and a polyurethane urea based on 1,4-butanediisocyanate. Polymer Bulletin,41(2), 131-138. doi:10.1007/s002890050343
[2] Wisse, E., Spiering, A. J., Leeuwen, E. N., Renken, R. A., Dankers, P. Y., Brouwer, L. A., . . . Meijer, E. W. (2006). Molecular Recognition in Poly(ε-caprolactone)-Based Thermoplastic Elastomers. Biomacromolecules,7(12), 3385-3395. doi:10.1021/bm060688t
[3] Minnen, B. V., Leeuwen, M. V., Kors, G., Zuidema, J., Kooten, T. V., & Bos, R. (2007). In vivo resorption of a biodegradable polyurethane foam, based on 1,4-butanediisocyanate: A three-year subcutaneous implantation study. Journal of Biomedical Materials Research Part A,85A(4), 972-982. doi:10.1002/jbm.a.31574
[4] Hafeman, A. E., Li, B., Yoshii, T., Zienkiewicz, K., Davidson, J. M., & Guelcher, S. A. (2008). Injectable Biodegradable Polyurethane Scaffolds with Release of Platelet-derived Growth Factor for Tissue Repair and Regeneration. Pharmaceutical Research,25(10), 2387-2399. doi:10.1007/s11095-008-9618-z
[5] Guan, J., Fujimoto, K. L., Sacks, M. S., & Wagner, W. R. (2005). Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials,26(18), 3961-3971. doi:10.1016/j.biomaterials.2004.10.018
[6] Kavlock, K., Pechar, T., Hollinger, J., Guelcher, S., & Goldstein, A. (2007). Synthesis and characterization of segmented poly(esterurethane urea) elastomers for bone tissue engineering. Acta Biomaterialia,3(4), 475-484. doi:10.1016/j.actbio.2007.02.001
[7] Yin, J. (2012). Lysine based amorphous polyurethanes decorated witn pendant bio-active groups Groningen: s.n.
[8] Santerre, J., Woodhouse, K., Laroche, G., & Labow, R. (2005). Understanding the biodegradation of polyurethanes: From classical implants to tissue engineering materials. Biomaterials,26(35), 7457-7470. doi:10.1016/j.biomaterials.2005.05.079
[9] Guelcher S, Srinivasan A, Hafeman A, Gallagher K, Doctor J, Khetan S, McBride S, Hollinger J. Synthesis, in vitro degradation, and mechanical properties of two-component poly (ester urethane)urea scaffolds: effects of water and polyol composition. Tissue Eng. 2007;13:2321–2333
[10] Guelcher, S. A., Gallagher, K. M., Didier, J. E., Klinedinst, D. B., Doctor, J. S., Goldstein, A. S., . . . Hollinger, J. O. (2005). Synthesis of biocompatible segmented polyurethanes from aliphatic diisocyanates and diurea diol chain extenders. Acta Biomaterialia,1(4), 471-484. doi:10.1016/j.actbio.2005.02.007
[11] Zhang, X., Battiston, K., Mcbane, J., Matheson, L., Labow, R., & Santerre, J. P. (2016). Design of biodegradable polyurethanes and the interactions of the polymers and their degradation by-products within in vitro and in vivo environments. Advances in Polyurethane Biomaterials, 75-114. doi:10.1016/b978-0-08-100614-6.00003-2