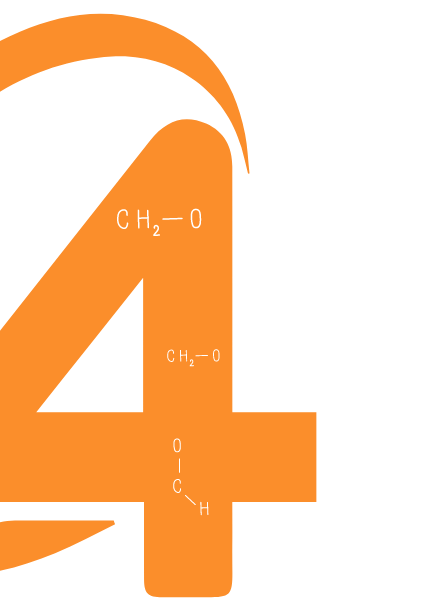- Homepage
- Services (ISO 13485)
Services (ISO 13485)
Common issues during R&D phase & file registration and production- Uncontrolled raw materials with no established specs.
- Compliant material specs. But Uncontrolled suppliers: Large suppliers such as Sigma or Merck
- Foreign suppliers:
- Suppliers without QMS:
- No counter samples/ retain samples
- No stability data on raw materials
- Scalability:
- Uncontrolled transport
- No monitored storage
- First or second supplier.
- Chemicals and other raw materials for your pharmaceutical or medical application.
- Selected suppliers
- Guarantee your specifications of your materials
- Third party analytical analysis
- Annual audit reports on suppliers
Rather contact us personally?

Sjonni, from origin a process engineer with more than 30 years’ experience in marketing and sales in the research, chemical and pharma industry.

Joris, a biomedical engineer specialized in organic chemistry with a strong background in quality and regulatory affairs.
Tailored packaging



Tailored volume or weight
Looking for tailored packaging solutions in the chemical industry? Whether you need specific volume requirements for each packaging unit, we provide flexible, reliable options. Our expertise ensures that your chemicals are packaged efficiently, complying with industry standards. From small to large quantities, we deliver custom packaging solutions that meet your unique volume needs. Optimize your supply chain with our safe, high-quality packaging services designed to protect your products and maintain their integrity. Contact us today to learn how we can help with your specific packaging volume requirements in the chemical industry.
Fields in which we can support
Stability studies
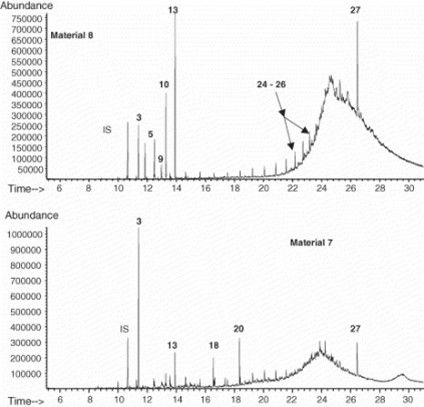
Extractables & Leachables
Retain samples
Our change notification program
Ensure compliance and minimize risks with our Change Notification Program. Stay informed about critical updates to products, processes, or regulations affecting your operations. Our program provides timely notifications, helping you adapt quickly and maintain uninterrupted workflows. Partner with us to stay ahead of industry changes and ensure continuous compliance.