Application: PEG 1000 for Pharmaceutical or Medical applications.
Synomyns: Poly(ethylene glycol), Polyethylene glycol 1000, Polyglycol, Polyethylene oxide, Polyoxy ethylene, PEG 1000, PEG
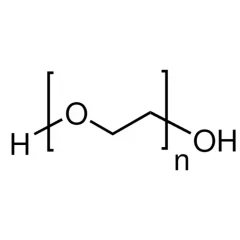
Linear formula: H(OCH2CH2)nOH
| Cat nr | Stock | Quantity (gr) | Price |
| t.b.d. | In stock | 5 kg | €150,-/kg |
**Pricing disclaimer
PEG-1000, or polyethylene glycol 1000, is a type of polyethylene glycol with a molecular weight around 1000 g/mol. It is a polymer compound composed of repeating ethylene glycol units. PEG-1000 is a water-soluble, odorless, and tasteless substance that has a variety of applications across different industries.
Drug Solubilization: PEG 1000 is often utilized as a solubilizing agent in pharmaceutical formulations. It can improve the solubility and bioavailability of poorly soluble drugs by forming stable complexes or enhancing their dispersibility in aqueous solutions. This property makes PEG 1000 particularly valuable in the formulation of oral, topical, and parenteral dosage forms.
Ointments and Creams: PEG 1000 serves as a base ingredient in various topical formulations such as ointments, creams, and gels. It helps to impart emollient and moisturizing properties, enhances spreadability, and provides a smooth texture to the product. These characteristics make PEG 1000 suitable for dermatological applications, including wound healing, skin hydration, and drug delivery through the skin.
Excipient in Pharmaceuticals: PEG 1000 is commonly used as an excipient in pharmaceutical formulations. It acts as a carrier or vehicle for active pharmaceutical ingredients (APIs) in solid, liquid, and semi-solid dosage forms. PEG 1000 can improve the stability, homogeneity, and shelf-life of pharmaceutical products, contributing to their overall quality and efficacy.
Laxatives: PEG 1000 is a key component in certain laxative formulations, particularly those used for bowel preparation before medical procedures such as colonoscopy or surgery. When combined with electrolytes, PEG 1000 forms solutions that induce osmotic laxative effects, facilitating the evacuation of the bowel contents. These formulations are preferred for their effectiveness, tolerability, and minimal systemic absorption.
Parenteral Formulations: PEG 1000 is used in the preparation of parenteral formulations such as injectable solutions and suspensions. It can serve as a stabilizer, cryoprotectant, or viscosity-modifying agent in these formulations, ensuring the compatibility, safety, and efficacy of the administered drug.
Biomedical Research: PEG 1000 is employed in various biomedical research applications, including cell culture, protein purification, and biotechnology. It can facilitate cell fusion, protein crystallization, and the modification of biomolecules, making it a versatile tool in laboratory settings.
Overall, the pharmaceutical applications of PEG 1000 highlight its versatility, safety, and efficacy in drug delivery, formulation, and biomedical research. However, it’s important to consider potential regulatory requirements, compatibility with other excipients, and patient-specific factors when using PEG 1000 in medical or pharmaceutical applications.
Polyethylene glycol (PEG) 1000, like other PEGs, is generally considered to be biodegradable under certain conditions. Biodegradability refers to the ability of a substance to be broken down into simpler compounds by microorganisms such as bacteria and fungi, ultimately resulting in the conversion of the substance into carbon dioxide, water, and biomass.
Polyethylene glycol (PEG) can contain impurities that arise during its production process or from storage conditions. These impurities can vary depending on the manufacturing method, starting materials, and purification processes used. Here are some common impurities found in PEG:
Ethylene Glycol: Ethylene glycol is a precursor in the synthesis of PEG and can be present as an impurity if not adequately removed during manufacturing. Ethylene glycol is toxic and can pose health risks if ingested or absorbed through the skin.
Diethylene Glycol: Diethylene glycol is another potential impurity in PEG products. It may form during the manufacturing process or as a degradation product of ethylene glycol. Diethylene glycol is also toxic and has been associated with serious health issues, including kidney and liver damage.
Higher Molecular Weight PEGs: PEG production processes can sometimes result in the formation of higher molecular weight PEGs as impurities. These higher molecular weight species may affect the properties of the final PEG product and its suitability for specific applications.
Oxidation Products: PEGs can undergo oxidative degradation, especially when exposed to elevated temperatures, light, or air. Oxidation products such as aldehydes and peroxides may form as impurities in PEG-containing products. These impurities can affect the stability and safety of the product.
Residual Catalysts: Some PEG synthesis methods involve the use of catalysts such as acids or bases. Residual catalysts left in the product can act as impurities and may affect the chemical properties of the PEG.
Residual Solvents: Solvents used during the manufacturing or purification process may remain as impurities in the final PEG product. These residual solvents must be carefully controlled to ensure product safety and compliance with regulatory standards.
Heavy Metals: PEGs can sometimes contain trace amounts of heavy metal impurities, which may originate from raw materials, equipment, or processing aids used during manufacturing.
It’s important for manufacturers to implement robust quality control measures to minimize impurities in PEG products and ensure their safety and purity. Regulatory agencies such as the FDA (Food and Drug Administration) provide guidelines and standards for the acceptable levels of impurities in pharmaceutical and medical-grade PEG products. Additionally, users should carefully review product specifications and certificates of analysis to assess the purity and quality of PEG materials.
Polyethylene glycol (PEG) is generally considered safe when used as directed and in accordance with established guidelines. It has a long history of use in various industries, including pharmaceuticals, cosmetics, food, and industrial applications. Here are some reasons why polyethylene glycol is considered safe:
Biocompatibility: PEG is biocompatible, meaning it is well-tolerated by living organisms, including humans. It is non-toxic and does not cause harm when ingested, applied to the skin, or administered in medical formulations. This property makes PEG suitable for use in a wide range of products intended for human consumption or contact.
Low Toxicity: PEG has low acute toxicity, meaning it does not pose significant health risks at typical exposure levels. However, like any substance, excessive ingestion or exposure to high concentrations of PEG may lead to adverse effects such as gastrointestinal discomfort or allergic reactions in some individuals.
Inertness: PEG is chemically inert, meaning it does not react with other substances under normal conditions. This inertness contributes to its stability and compatibility with a wide range of materials and formulations. It also reduces the likelihood of PEG causing chemical reactions or interactions that could lead to adverse effects.
Approved by Regulatory Agencies: PEG and its derivatives are regulated by health authorities such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other regulatory agencies worldwide. These agencies review scientific data on the safety and efficacy of PEG-containing products and establish guidelines and standards for their use in various applications.
Well-Studied: PEG has been extensively studied for its safety profile in both animal and human studies. Research has shown that PEG is not carcinogenic, mutagenic, or teratogenic at typical exposure levels. However, some concerns have been raised about the potential for allergic reactions or sensitization in susceptible individuals.
Wide Usage: PEG is widely used in pharmaceuticals, cosmetics, food, and industrial applications without significant reports of adverse effects when used appropriately. Its versatility and safety make it a preferred ingredient in many consumer products.
Polyethylene glycol (PEG) is a polyether compound with a wide range of applications, and it comes in various types distinguished by their molecular weights and properties. Here are some common types of polyethylene glycol:
Low Molecular Weight PEGs (LMW-PEGs): These PEGs typically have molecular weights ranging from around 200 to 700 g/mol. They are often used as solvents, lubricants, and plasticizers in various industrial applications. LMW-PEGs are also utilized in pharmaceutical formulations, especially in oral medications and topical preparations.
Medium Molecular Weight PEGs (MMW-PEGs): MMW-PEGs have molecular weights ranging from approximately 1000 to 20,000 g/mol. They find applications in pharmaceuticals, cosmetics, and personal care products as solubilizing agents, emulsifiers, and thickeners. MMW-PEGs are often used in formulations such as creams, ointments, and oral suspensions.
High Molecular Weight PEGs (HMW-PEGs): HMW-PEGs have molecular weights exceeding 20,000 g/mol. They are employed in various industrial and biomedical applications, including drug delivery systems, biomaterials, and tissue engineering. HMW-PEGs are valued for their biocompatibility, water solubility, and ability to modify the properties of biomolecules.
Monodisperse PEGs: Monodisperse PEGs are characterized by a narrow molecular weight distribution, resulting in uniform polymer chains with consistent properties. These PEGs are often used in research and biotechnology applications, such as protein conjugation and nanoparticle synthesis, where precise control over molecular structure is crucial.
Functionalized PEGs: Functionalized PEGs contain chemical moieties or groups attached to the polymer backbone, imparting specific properties or functionalities. Examples include PEGs modified with reactive end groups (e.g., hydroxyl, amino, carboxyl) for conjugation chemistry, PEGylated lipids for drug delivery, and PEG derivatives with targeting ligands for selective cell or tissue interactions.
Methoxy PEGs (mPEGs): Methoxy PEGs are PEG derivatives in which one or both terminal hydroxyl groups are substituted with methoxy (–OCH3) groups. These modifications enhance the stability and circulation half-life of therapeutic agents by reducing immunogenicity and enzymatic degradation. mPEGs are extensively used in the PEGylation of proteins, peptides, and nanoparticles for drug delivery applications.
Polyethylene Glycol Dimethyl Ethers (PEG-DMEs): PEG-DMEs are PEG derivatives in which the ethylene oxide units are connected by dimethyl ether (-OCH3) linkages instead of the typical ether (-O-) linkages. These compounds exhibit improved solubility in nonpolar solvents and are utilized in applications such as phase transfer catalysts and specialty coatings.
Polyethylene glycol (PEG) is soluble in both water and many organic solvents. Its solubility depends on factors such as molecular weight, temperature, and the nature of the solvent. Here are some details about PEG solubility:
Water: PEG is highly soluble in water, which makes it particularly useful in aqueous formulations such as pharmaceuticals, cosmetics, and personal care products. Even at high molecular weights, PEGs exhibit excellent water solubility, allowing them to be easily incorporated into water-based formulations.
Organic Solvents: PEGs are also soluble in many organic solvents, including alcohols (e.g., ethanol, methanol), ketones (e.g., acetone), ethers (e.g., diethyl ether), and chlorinated solvents (e.g., chloroform). The solubility of PEG in organic solvents depends on factors such as molecular weight, temperature, and the polarity of the solvent.
Temperature Dependence: The solubility of PEG can vary with temperature. Generally, higher temperatures increase the solubility of PEG in both water and organic solvents. However, there may be exceptions depending on the specific PEG grade and solvent system.
Hydrophobic Interactions: Despite its excellent water solubility, some higher molecular weight PEGs (e.g., PEG 8000 and above) may exhibit limited solubility in water at room temperature due to hydrophobic interactions between polymer chains. However, heating or stirring can often enhance solubility.
Salts and Additives: The addition of salts or other additives can affect the solubility of PEG in water or organic solvents. For example, the addition of salts such as sodium chloride or potassium phosphate can influence the phase behavior of PEG solutions and alter its solubility properties.
Cosolvency: In some cases, cosolvents or surfactants may be used to enhance the solubility of PEG in certain solvent systems. These additives can help overcome solubility limitations and improve the compatibility of PEG with other ingredients in formulations.
Overall, the solubility of polyethylene glycol in water and organic solvents makes it a versatile and widely used polymer in various industries, including pharmaceuticals, cosmetics, food, and industrial applications. Its compatibility with different solvents allows for the formulation of diverse products with desired properties and performance characteristics
The PEG 1000 offered by 4MedChem is allowed to be used in products that are FDA registered. We will support in all required documentation needed for you final product file.