- Homepage
- All Products
- Polymers - Biodegradable & Biocompatible
- D-Lactide - CAS 13076-17-0
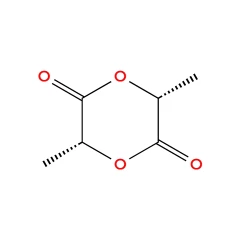
D-Lactide - CAS 13076-17-0
Product specifications
Purity: >99.5%
Name: L-3,6-Dimethyl-1,4-dioxane-2,5-dione
CAS No: 13076-17-0
Appearance: white particle
Molecular Formula: C₆H₈O₄
Molecular Weight: 144.13 g/mol
Appearance: White crystalline solid
Melting Point: ~96–98°C
Solubility: Soluble in organic solvents (e.g., chloroform, ethyl acetate, acetone); insoluble in water.
Optical Activity: Dextrorotatory (Rotates plane-polarized light to the right)
Price & Availability
Prices are available upon request with our account manager for this product: Sjonni@4MedChem.com
D-Lactide: The Enantiomer of L-Lactide for Biodegradable Polymers
D-Lactide is one of the two optically active enantiomers of lactide, derived from D-lactic acid. It is a cyclic di-ester (dimer) and serves as a key precursor in the production of poly-D-lactic acid (PDLA), a biodegradable and biocompatible polymer used in medical, pharmaceutical, and packaging applications.
Synthesis & Production
D-Lactide is synthesized through the polymerization and controlled cyclization of D-lactic acid, typically obtained from bacterial fermentation of renewable sources like corn starch or sugarcane.
The production process involves:
- Lactic Acid Formation → Microbial fermentation of D-lactic acid.
- Oligomer Formation → Condensation of D-lactic acid to form low-molecular-weight prepolymers.
- Depolymerization & Cyclization → Heating under vacuum to form high-purity D-lactide.
D-Lactide undergoes ring-opening polymerization (ROP) using catalysts such as tin(II) octoate to produce PDLA (poly-D-lactic acid).
Applications
Biodegradable Polymers (PDLA)
-
- Used in biomedical implants, biodegradable packaging, and sustainable plastics.
- When combined with PLLA (poly-L-lactic acid), it forms stereocomplex PLA, which has enhanced thermal and mechanical properties.
Medical Applications
-
- Used in resorbable sutures, orthopedic implants, and scaffolds for tissue engineering.
- PDLA degrades slower than PLLA, making it suitable for long-term applications.
High-Performance PLA (Stereocomplex PLA)
-
- Mixing PDLA with PLLA results in stereocomplex PLA, which has a higher melting point (~230°C vs. ~180°C for PLA alone).
- This enhances thermal stability, mechanical strength, and crystallinity, making it useful in automotive, electronics, and industrial applications.
Drug Delivery Systems
-
- Used in controlled drug release formulations, ensuring biodegradability and biocompatibility.
Comparison: D-Lactide vs. L-Lactide vs. DL-Lactide
| Property | D-Lactide | L-Lactide | DL-Lactide |
|---|---|---|---|
| Optical Activity | Dextrorotatory (+) | Levorotatory (-) | Racemic (±) |
| Melting Point | ~96–98°C | ~96–98°C | ~124–126°C |
| Polymer Type | Crystalline PDLA | Crystalline PLLA | Amorphous PDLLA |
| Biodegradability | Slow | Slow | Faster |
| Mechanical Strength | High (when blended with PLLA) | High | Low |
Advantages of D-Lactide
- Enhances thermal and mechanical properties when blended with PLLA (stereocomplex PLA).
- Longer degradation time, making it ideal for medical implants and drug delivery systems.
- Biocompatible and biodegradable, reducing environmental impact.
Challenges
- Requires specific fermentation processes to obtain high-purity D-lactic acid.
- Slower degradation than racemic PLA, which may not be ideal for short-term applications.
Conclusion
D-Lactide is a valuable monomer for biodegradable polymers, particularly in high-performance applications where thermal stability and strength are required. It plays a crucial role in medical, packaging, and sustainable material industries, especially when blended with PLLA to form stereocomplex PLA, offering superior mechanical properties compared to traditional PLA.