- Homepage
- All Products
- Polymers - Biodegradable & Biocompatible
- Hexafluoro isopropanol - Cas 920-66-1 (99,9%)
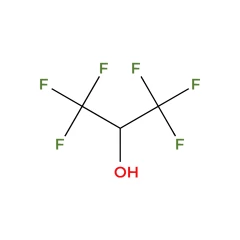
Hexafluoro isopropanol - Cas 920-66-1 (99,9%)
Product specifications
Purity: 99,9%
Name: hexafluoro isopropanol
Cas: 920-66-1
Synonyms: HFIP, HFP, Hexafluoroisopropanol, 1,1,1,3,3,3-Hexafluoro-2-propanol, Hexafluoroisopropyl alcohol
Chemical formula: C3H2F6O
Molar mass: 168.038 g·mol−1
Appearance: Colorless liquid
Density: 1.596 g/mL
Melting point: −3.3 °C (26.1 °F; 269.8 K)
Boiling point:58.2 °C (136.8 °F; 331.3 K)
Solubility in water: Miscible
Vapor pressure: 16 kPa at 20 °C
Viscosity: 1.65 cP at 20 °C
For bulk inquiries please contact: Sjonni@4MedChem.com
Price & Availability
| Cat nr | Stock | Quantity | Price |
|---|---|---|---|
| 80803040025 (40 gram) | In stock | 25 ml | €150 |
| 80803040100 (160 gram) | In stock | 100 ml | €210 |
| 80803040500 (800 gram) | In stock | 500 ml | €495 |
Hexafluoroisopropanol
Hexafluoroisopropanol (HFIP), also known as 1,1,1,3,3,3-hexafluoro-2-propanol, is a fluorinated alcohol with the chemical formula (CF₃)₂CHOH. This colorless, volatile liquid is recognized for its distinctive properties, making it valuable in various scientific and industrial applications.
Production and Chemical Properties
HFIP is synthesized through the hydrogenation of hexafluoroacetone. It is a polar solvent with strong hydrogen-bonding capabilities, a high dielectric constant of 16.7, and an acidity (pKa) of 9.3, comparable to phenol. These characteristics contribute to its effectiveness in stabilizing ionic species and facilitating various chemical reactions.
Applications in Organic Synthesis hexafluoro isopropanol
In organic chemistry, hexafluoro isopropanol is esteemed for its role as a versatile solvent. Its unique properties enhance the reactivity of certain reagents and stabilize reaction intermediates. For instance, HFIP has been utilized to promote the reactivity of hydrogen peroxide in Baeyer–Villiger oxidations and to facilitate Lewis acid-catalyzed ring-opening reactions of epoxides.
Use in Biochemistry
Beyond organic synthesis, hexafluoro isopropanol is employed in biochemistry for its ability to solubilize peptides and disrupt β-sheet protein aggregates. This makes it a valuable tool in protein studies, particularly in the analysis and manipulation of amyloid fibrils and other protein assemblies.
Role in Anesthetics
Medically, hexafluoro isopropanolis recognized as both a precursor and the primary metabolite of the inhalation anesthetic sevoflurane. Upon administration, sevoflurane is metabolized into HFIP and formaldehyde. HFIP is inactive and non-genotoxic; once formed, it is rapidly conjugated with glucuronic acid and eliminated as a urinary metabolite.
Safety Considerations hexafluoro isopropanol
While hexafluoro isopropanole xhibits low acute toxicity, it is a strong irritant to skin and eyes. Animal studies have indicated potential adverse effects on fertility, leading to its classification as a reproductive toxicity category 2 substance. Proper handling and safety protocols are essential when working with HFIP to mitigate these risks.
References
https://www.nature.com/articles/s41570-017-0088