HFIP (Hexafluoroisopropanol): The Versatile Fluorinated HFIP Solvent Transforming Science and Industry
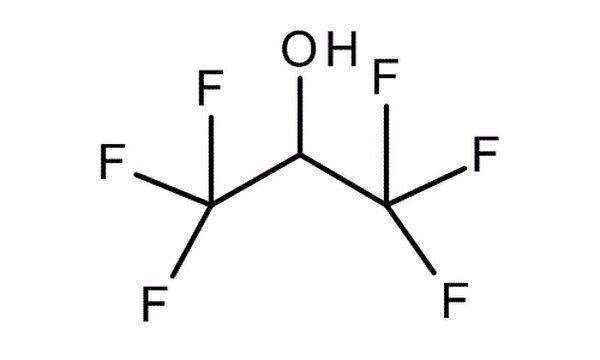
Hexafluoroisopropanol (HFIP), also known as 1,1,1,3,3,3-hexafluoro-2-propanol, is a unique fluorinated alcohol that has gained significant attention across multiple scientific and industrial domains. This volatile, colorless liquid offers a distinct combination of high polarity, hydrogen bonding capability, and chemical stability, making it a powerful solvent for various applications. From organic synthesis to biomaterials and pharmaceuticals, HFIP as a solvent plays a crucial role in advancing modern technologies.
Two qualities available at 4MedChem
| 99,9% | 99% |
| Click here to see the product | Click here to see the product |
 |
 |
|
Pricing:
|
Pricing:
|
Chemical Properties and Synthesis HFIP
HFIP is a highly polar protic solvent with a dielectric constant of approximately 16.7 and an acidity (pKa) of 9.3, similar to phenol. Its strong hydrogen bonding abilities allow it to dissolve a wide range of polymers and proteins, making it invaluable in materials science and biochemistry. HFIP is typically synthesized through the hydrogenation of hexafluoroacetone, a process that yields high purity and ensures stability under various conditions.
Applications in Science and Industry HFIP
1. Organic Synthesis and Catalysis
HFIP is widely used as a solvent in organic synthesis due to its ability to stabilize reactive intermediates and enhance reaction rates. It has been employed in:
-
Electrophilic fluorination reactions to introduce fluorine atoms into organic molecules.
-
Baeyer–Villiger oxidation, where it enhances the reactivity of hydrogen peroxide.
-
Cyclization reactions, particularly in the synthesis of heterocycles and pharmaceuticals.
2. Electrospinning and Polymer Processing
One of the most exciting applications of HFIP is in electrospinning, a technique used to create ultrafine nanofibers for biomedical and industrial applications. HFIP’s ability to dissolve proteins and synthetic polymers such as polycaprolactone (PCL) and poly(lactic-co-glycolic acid) (PLGA) makes it an essential solvent for producing biocompatible fibers used in tissue engineering, wound healing, and drug delivery systems.
3. Pharmaceutical and Biochemical Applications
HFIP is used extensively in protein and peptide research. It is particularly effective in:
-
Solubilizing and refolding proteins, making it valuable in structural biology.
-
Disrupting β-sheet structures in protein aggregates, which is beneficial for studying amyloid diseases such as Alzheimer’s.
-
Serving as a precursor and metabolite of anesthetics, such as sevoflurane, a widely used inhalation anesthetic.
Safety and Environmental Considerations HFIP
Despite its advantages, HFIP must be handled with care due to its strong solvent properties and potential toxicity. It can cause skin and eye irritation and has been classified as a Category 2 reproductive toxin. Proper safety protocols, including the use of gloves and fume hoods, are necessary when working with HFIP.
In terms of environmental impact, HFIP belongs to the class of per- and polyfluoroalkyl substances (PFAS), which are known for their persistence. However, since HFIP is produced and used in controlled environments, its environmental footprint is relatively low compared to other fluorinated compounds.
Conclusion
Hexafluoroisopropanol is a game-changing solvent with applications spanning chemistry, material science, and medicine. Its role in organic synthesis, electrospinning, and pharmaceutical research highlights its versatility and importance. As research continues, HFIP will likely remain a cornerstone in scientific advancements while being scrutinized for environmental sustainability and safety.
References HFIP Solvent
-
Nature Chemistry Reviews: "The Role of HFIP in Organic Reactions."
-
ACS Publications: "Electrospinning of Biodegradable Polymers Using HFIP."
-
Journal of Medicinal Chemistry: "HFIP as a Key Solvent in Protein Folding Studies."

