Waterborne polyurethane synthesized for leather with excellent wear and hydrolysis resistance enabled by bio-based poly(trimethylene carbonate) and l-lysine diisocyanate

Polyurethane (PU) synthetic leather is traditionally manufactured through dry transfer coating or wet processes, utilizing nonwoven fabrics as the base with PU resin as the coating, allowing it to mimic the texture of genuine leather. In recent years, waterborne polyurethane (WPU) has gained increasing attention in the synthetic leather industry due to its non-toxic nature and enhanced safety. However, the production of WPU remains heavily reliant on petrochemical resources, presenting challenges in terms of biodegradability, resource depletion, and environmental pollution. To address these concerns, the development of bio-based WPU (BWPU) using renewable resources has emerged as a significant trend in the industry.
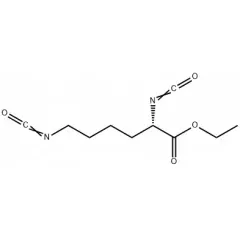
Current research in BWPU primarily focuses on substituting petroleum-derived polyols with biomass-based alternatives, including vegetable oil, lignin, and sugar-derived compounds. Despite these advancements, BWPU often exhibits inferior mechanical properties, necessitating the use of petroleum-based aromatic isocyanates such as 4,4-diphenylmethane diisocyanate (MDI) and toluene diisocyanate (TDI) to enhance performance. However, these aromatic isocyanates pose potential health risks. As a safer alternative, L-lysine diisocyanate (LDI), a bio-based diisocyanate, has gained attention for its effective reactivity with polyols and non-toxic degradation products. Recent studies have demonstrated the potential of LDI-based BWPU in various applications. For instance, Burcu Acik et al. developed a novel bio-based polyurethane (CA-PCL-PU) using cholic acid-based poly(ε-caprolactone) (CA-PCL) and LDI, resulting in high hydrophilicity and biodegradability. Similarly, Wang et al. synthesized biodegradable polyurethanes incorporating LDI, polyethylene glycol, and polyhydroxyalkanoates, exhibiting excellent biocompatibility suitable for culturing rat bone marrow stem cells. While LDI offers a promising alternative to aromatic isocyanates, BWPU formulated with LDI still faces challenges in terms of mechanical strength and hydrolysis resistance.
PU is a block copolymer composed of soft and hard segments, where the hard segments influence mechanical strength and crystallinity, while the soft segments contribute to hydrolytic stability and flexibility at low temperatures. The polyol chains forming the soft segments are crucial for achieving high-performance BWPU. Over the past decades, researchers have sought to improve BWPU’s mechanical properties and hydrolysis resistance by synthesizing bio-based polyols through the ring-opening polymerization of cyclic bio-monomers. Polylactide diols (PLA-OH), derived from lactide polymerization using small-molecule diol initiators, have been widely explored for BWPU synthesis. However, PLA-OH's high glass transition temperature and poor hydrolysis resistance significantly limit its industrial applications. Consequently, developing BWPU with superior mechanical properties and hydrolysis resistance remains a major challenge.
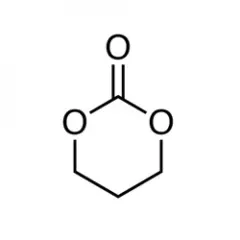
Advancements in bio-fermentation and isolation technologies have facilitated the production of bio-based trimethylene carbonate (TMC) monomers using 1,3-propanediol (bio-PDO) and dialkyl carbonates. Polytrimethylene carbonate (PTMC), synthesized through the ring-opening polymerization of TMC, is an aliphatic polycarbonate with a low glass transition temperature (Tg = -19 °C), low Young’s modulus (4.7 MPa), and high elongation at break (1250%). Recognized for its hydrophobicity and environmental compatibility, PTMC has been extensively studied for various applications. Hou et al. successfully synthesized bio-based P(TMC-co-DTC) through the copolymerization of TMC and 2,2'-dimethyltrimethylene carbonate (DTC), demonstrating excellent form stability. Additionally, Gielen et al. developed hybrid hydrogels for tissue regeneration using hyaluronic acid (HA) and hydrophobic PTMC, exhibiting suture retention strength comparable to natural vascular tissues. Although PTMC is predominantly applied in biomedical fields, its potential for broader industrial applications, including synthetic leather, is gaining interest.
Mengchem Xue et al. (2024) have prepared poly(trimethylene carbonate) diols (PTMC-OH) through ring-opening polymerization of trimethylene carbonate (TMC) and 1,4-butanediol (BDO) as an initiator. A series of linear bio-based waterborne polyurethanes (LBWPU) with different hard segment content were prepared using PTMC-OH as the bio-based polyols and LDI as the bio-based isocyanates, in which the hydrogen bonds content varied with the hard segment content. Moreover, the synergistic strategy of hydrogen bonds and chemical crosslinking structure was employed in crosslinked bio-based waterborne polyurethanes (CBWPU) using trimethylolpropane (TMP) as the crosslinking agent. The study has systematically investigated the effects of structure in BWPU on their thermal stability, hydrolytic stability and mechanical properties. Furthermore, the application of BWPU films as the coating for synthetic leather has also been investigated and discussed. This work provides a simple and effective method for the preparation of BWPU with high bio-based content and high performance, promoting the application of BWPU in the synthetic leather industry.
.... continued
Request full article at: joris@4MedChem.com
Mengchen Xue, Xiang Rao, Weihu Li, Jinghua Du, Wenhe Guo, Wangqin Zhou, Xiaoyu Dong, Le Wu, Guobing Zhang, Yunsheng Ding. 2024
|
In-stock Available |
In-stock Available |
 |
 |

